How Do Ro Drinking Water Systems Work?
If you’ve ever taken a sip from a glass of water that came from the home of someone with a reverse osmosis system, you know how pure and refreshing it tastes.
Or, perhaps you have concerns about water quality and want to make sure your family is drinking healthy water that reduces contaminants as much as possible.
Reverse osmosis (R.O.) drinking water truly is the purest choice for any home. It’s water the way nature intended us to drink it.
But how exactly do these systems work, and what do they do to your home’s water?
What is reverse osmosis?

Osmosis is defined as the process of molecules passing through a semi-permeable membrane from a less-concentrated solution into a more-concentrated solution.
An example or osmosis from nature is the roots of plants drawing water from the soil.
Reverse osmosis is simply the opposite of that process.

There’s a bit more to the process when using a reverse osmosis system to purify drinking water.
If you’ve ever seen an R.O. system, you’ve likely noticed the two or three big tanks setup with it. They’re quartz sand filter, carbon filter(Softening filter if there is three of them) to remove larger sediment, including some dissolved solids, and help reduce chlorine

in order to protect the RO membrane. Of course there’ll also be a smaller steel tank just ahead of the RO membrane which is called the precision(micron) filter. It’s also to conserve the membrane which can get clogged by excess sediment or damaged by exposure to too much chlorine that you’ll find in municipal water.
Reverse osmosis works best when you start with good water and then make it great. That’s why you should never use a reverse osmosis system with hard water unless it is under 10 grains per gallon. We often recommend having a water softener tank(the third tank mentioned above)installed before installing an R.O. system. Scale buildup from hard water can damage these systems in the same way they damage other appliances.
Following the initial filtration comes the real magic of an R.O. system.

Your water is forced through the semi-permeable membrane under pressure. The membrane is a synthetic plastic material that allows the passage of water molecules. However, sodium, chlorine, and calcium as well as larger molecules like glucose, urea, bacteria and viruses cannot pass.
We have reverse osmosis drinking water systems that are tested and certified for reduction of:
· lead
· arsenic
· copper
· nitrates and nitrites
· chromium (hexavalent & trivalent)
· selenium
· fluoride
· radium
· barium
· cadmium
· cyst (cryptosporidium)
· total dissolved solids (TDS)
This type of membrane is resistant to bacteria breakdown and has a high rejection rate of 95 to 97 percent on average. TFC membranes are not chlorine-resistant, which is why a carbon prefilter is used.Before your home’s water is ready to drink, it goes through a second carbon filter (or post filter), which removes any remaining contaminants in the unlikely case they slipped past the membrane.
Then the water fills up a storage tank where it waits until you’re ready to use it.
Finally, there’s the in-line activated carbon filter, which gives your water one last polish as it comes out your faucet. This is used to remove any remaining odors or flavors that may come from the system hoses or the holding tank.

The polish is a “just in case” step to make sure the water you drink tastes incredibly fresh!



 Containerized Seawater Desalination System
Containerized Seawater Desalination System Seawater Desalination System
Seawater Desalination System Small/Marine Watermaker Systems
Small/Marine Watermaker Systems RO+EDI Ultrapure Water System
RO+EDI Ultrapure Water System Media Filter
Media Filter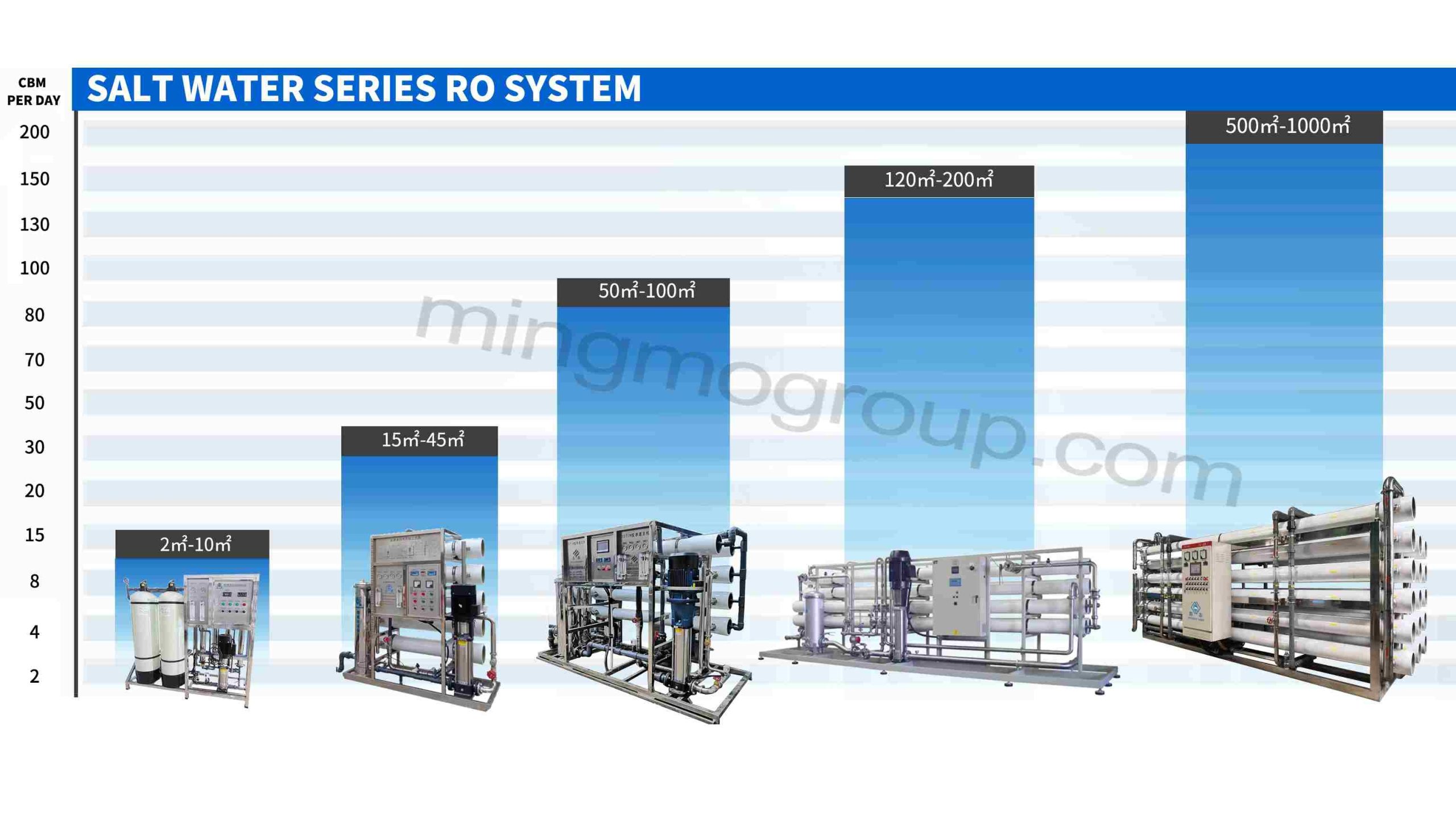 Brackish Water Reverse Osmosis Treatment System
Brackish Water Reverse Osmosis Treatment System Large RO Desalination Machine
Large RO Desalination Machine Ultrafiltration System vs. Reverse Osmosis System: Which One Should You Choose?
Ultrafiltration System vs. Reverse Osmosis System: Which One Should You Choose?