What is Reverse Osmosis?
Reverse Osmosis is a technology that is used to remove a large majority of contaminants from water by
pushing the water under pressure through a semi—permeable membrane. This paper will attempt to explain the basics in simple terms that should leave the reader with a better overall understanding of Reverse Osmosis technology and its applications.
Reverse osmosis, commonly referred to as RO, is a process where you remove a large portion of dissolved solids and other contaminants from water by forcing the water through a semi-permeable reverse osmosis membrane.
A semi—permeable membrane is a membrane that will allow some atoms or molecules to pass but not others. A simple example is a screen door. It allows air molecules to pass through but not pests or anything larger than the holes in the screen door. The pores are big enough to let water vapor through, but small enough to prevent liquid water from passing.
Reverse Osmosis is the process of Osmosis in reverse. Whereas Osmosis occurs naturally without energy required, to reverse the process of osmosis you need to apply energy to the more saline solution. A reverse osmosis membrane is a semi—permeable membrane that allows the passage of water molecules.
But not most of the dissolved salts, organics, bacteria, and pyrogens. However, you need to ‘push’ the water through the reverse osmosis membrane by applying pressure that is greater than the naturally occurring osmotic pressure.




 Containerized Seawater Desalination System
Containerized Seawater Desalination System Seawater Desalination System
Seawater Desalination System Small/Marine Watermaker Systems
Small/Marine Watermaker Systems RO+EDI Ultrapure Water System
RO+EDI Ultrapure Water System Media Filter
Media Filter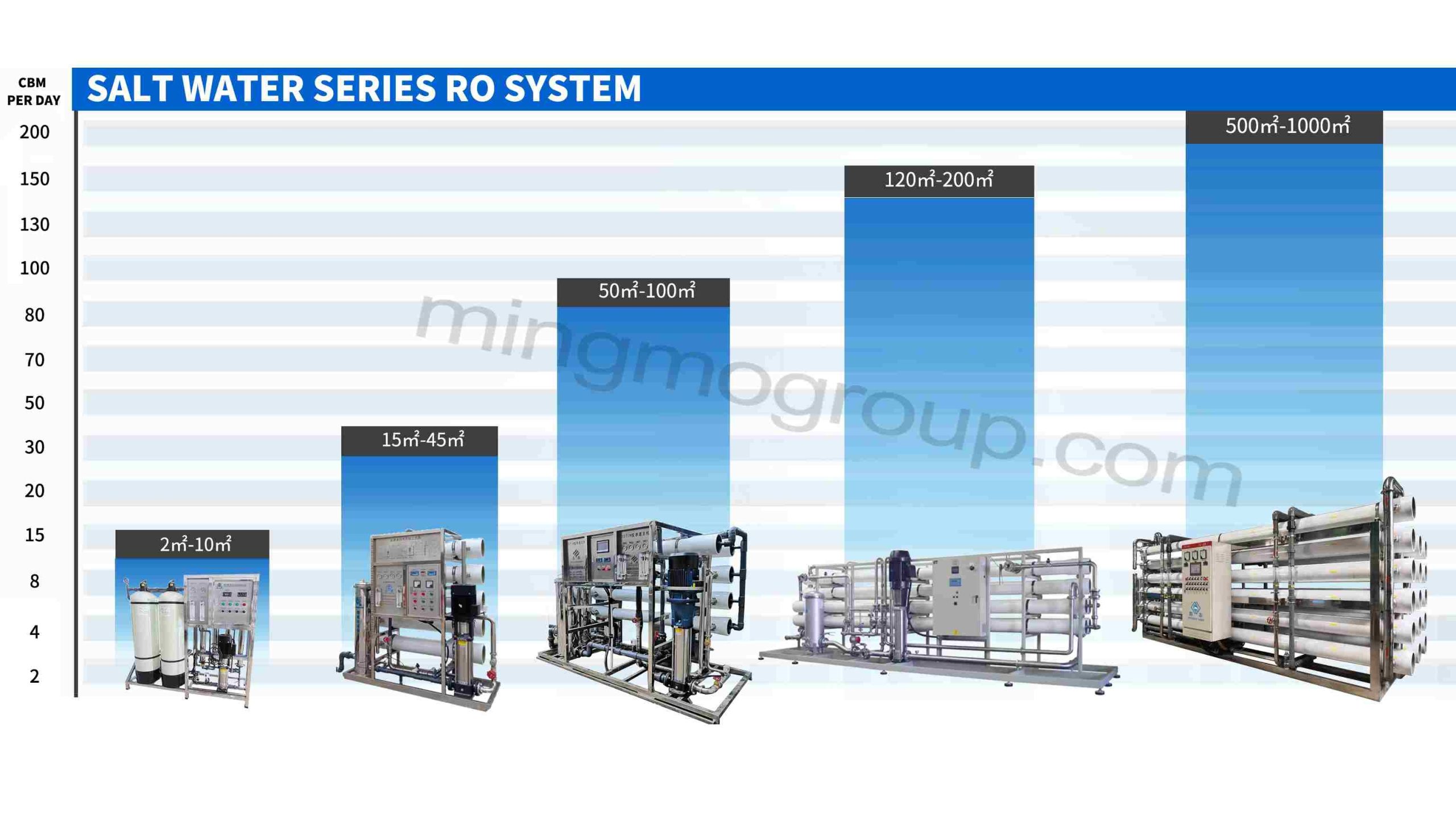 Brackish Water Reverse Osmosis Treatment System
Brackish Water Reverse Osmosis Treatment System Large RO Desalination Machine
Large RO Desalination Machine Ultrafiltration System vs. Reverse Osmosis System: Which One Should You Choose?
Ultrafiltration System vs. Reverse Osmosis System: Which One Should You Choose?