What’s resin in water treatment?
Water treatment resin is divided into cationic resin and anionic resin, cationic resin is subdivided into sodium type and hydrogen type, sodium type resin will exchange calcium and magnesium ions in water into sodium ions, so that the water becomes soft. Hydrogen resin is the exchange of calcium and magnesium ions in water into hydrogen ions to soften the water. Anionic resin containing replaceable hydroxide ions, can replace the acid ions in the water, and the use of anionic resin and hydrogen cation resin can turn the water into pure water.
In the water treatment industry, ion exchange is the reaction between ions in water and ions on ion exchange resin.

Physical properties
Particle size and related physical properties of ion exchange resin have great influence on its work and performance.
Resin particle size
The size of ion-exchange resins, which are usually made into small beads, is also important. Resin particles are smaller, the reaction speed is larger, but the resistance of fine particles to liquid through the larger, need higher working pressure; Especially the high viscosity of concentrated sugar liquid, this effect is more significant. Therefore, the size of resin particles should be selected appropriately. If the resin particle size is less than 0.2mm(about 70 mesh), it will significantly increase the resistance through the fluid, reducing the flow rate and production capacity.
The determination of resin particle size is usually wet screening method, the resin in full water absorption expansion after screening, accumulated in 20, 30, 40, 50…… The amount of remaining particles on the mesh mesh to 90% can pass through its corresponding sieve diameter, known as the “effective particle size” of the resin. The effective particle size of most common resin products is between 0.4 and 0.6mm.
Density of resin
The drying density of resin is called true density. The weight of wet resin per unit volume (including intergranular voids) is called apparent density. The density of the resin is related to its crosslinking degree and the properties of the exchange groups. In general, the density of resins with high cross-linking degree is higher, the density of strongly acidic or alkaline resins is higher than that of weak acids or bases, and the density of macroporous resins is lower.
Solubility of resin
The ion exchange resin shall be insoluble. But the resin in the synthesis process of inclusion of low degree of polymerization, and the resin decomposition of the material, will be dissolved during the operation. The resin with lower crosslinking degree and more active groups has a greater tendency to dissolve.
Expansion of resin
Ion exchange resin contains a large number of hydrophilic groups, and water contact that water expansion. When the ion transformation in the resin, such as cationic resin from H+ to Na+, negative resin from Cl- to OH-, due to the increase of ion diameter and expansion, increase the volume of the resin. In general, resins with low crosslinking degree have higher swelling degree. The expansion of resin must be considered in the design of ion exchange device to adapt to the volume change caused by ion conversion in resin during production operation.
Durability of resin
Resin particles when used transfer, friction, expansion and contraction changes, long-term use will have a small amount of loss and broken, so the resin should have higher mechanical strength and wear resistance. Generally, resins with low crosslinking degree are more likely to fracture, but the durability of resins depends more on the uniformity and strength of the crosslinking structure. Such as macroporous resin, with a high degree of cross-linking, stable structure, repeated regeneration.
Cationic resin
This kind of resin contains a large number of strongly acidic groups, such as sulfonic acid-so3H, easy to dissociate H+ in solution, so it is highly acidic. After resin dissociation, the body containing negative groups, such as SO3-, can adsorb other cations in the binding solution. These two reactions exchange H+ in the resin with cations in the solution. Strong acidic resins have strong dissociation ability and can dissociate and produce ion exchange in acidic or alkaline solutions. Resin in use after a period of time, to carry out regeneration treatment, that is, chemicals to make the ion exchange reaction in the opposite direction, so that the functional groups of the resin back to the original state, for use again. Such as the above cationic resin is treated with strong acid regeneration, at this time the resin released by adsorption of cation, and then combined with H+ and restore the original composition.



 Containerized Seawater Desalination System
Containerized Seawater Desalination System Seawater Desalination System
Seawater Desalination System Small/Marine Watermaker Systems
Small/Marine Watermaker Systems RO+EDI Ultrapure Water System
RO+EDI Ultrapure Water System Media Filter
Media Filter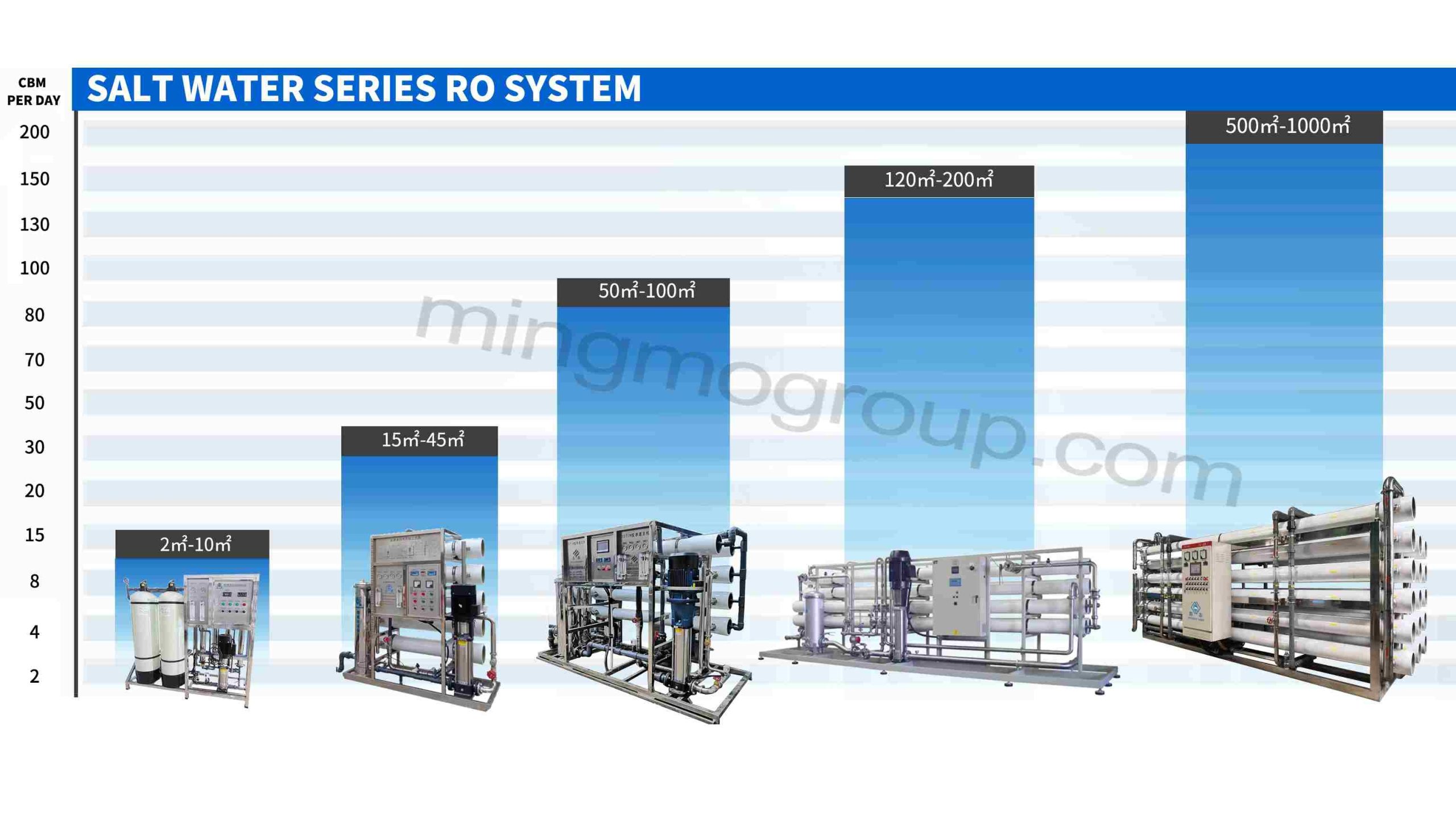 Brackish Water Reverse Osmosis Treatment System
Brackish Water Reverse Osmosis Treatment System Large RO Desalination Machine
Large RO Desalination Machine Ultrafiltration System vs. Reverse Osmosis System: Which One Should You Choose?
Ultrafiltration System vs. Reverse Osmosis System: Which One Should You Choose?